Soap makers often assume the purity of the alkali (NaOH or KOH) in their soaping stash is 100% pure, but the purity of real-life NaOH or KOH is never 100%. Even if the purity is very high when the alkali is newly purchased, the purity can drop as time passes, sometimes by quite a bit.
NaOH or KOH does not "go bad" simply because of time, however. What makes either one go bad is exposure to water and carbon dioxide (CO2) in the air. If you can keep an alkali properly protected, it can last for centuries. If you don't protect it from water and CO2 (or other things it wants to react with), it will lose a lot of its purity within mere hours or days.
When NaOH or KOH absorbs water from the air, it gains water weight. One gram of NaOH that is clumpy from absorbing water is not one gram of pure NaOH. Some of that weight will be useless water.
When NaOH or KOH also absorbs CO2 from the air, NaOH will react with the CO2 and become sodium carbonate (washing soda) and KOH reacts with CO2 to become potassium carbonate. The carbonates are alkali chemicals but they don't easily react with fats to make soap. One gram of NaOH that's contaminated with sodium carbonate is not one gram of pure NaOH.
If you base your recipe on using 100% pure NaOH or KOH (and many online soap recipe calculators make this assumption!), but you soap with NaOH or KOH that has a lower purity, the fats will not fully saponify. You can expect a range of results from "the soap looks fine but if I could measure the superfat, I would find it is is overly high" to "the soap will not come to trace -- it has stayed soupy for hours!"
Just because NaOH or KOH is less than 100% pure does not mean it should not be used to make soap. But how can we tell what the purity really is?
A rough check of NaOH purity is to make a 50% NaOH solution using room temperature water and your NaOH. Make enough lye solution as you normally would for making a regular batch of soap -- do not make just a tiny amount. Check the temperature of the freshly made mixture as soon as possible after the NaOH is completely dissoved. The temperature should be at least 180 F / 82 C and ideally even higher.
When I made a 50% NaOH solution recently, the temperature of the freshly made solution reached 216 F / 102 C. I took that as an indicator that my NaOH was fairly pure and should work well for making soap.
If the temperature of the freshly made solution does not reach 180 F / 82 C, the NaOH may not be very strong and you might need to use more NaOH to compensate for it not being as pure.
You can also do a more accurate check of NaOH purity. Once you have a number for the purity, that information can be used to adjust the alkali weight so enough actual alkali is used to make good soap.
A rigorous method for kitchen chemists would be the procedure in Kevin Dunn's book Scientific Soapmaking. (1) Based on information given in a presentation by Kevin Dunn (2), here is a somewhat less accurate, but easier method for testing the purity of your NaOH or KOH --
Video tutorial of this purity check (YouTube)
Step-by-step instructions and the math behind the formulas (PDF)
Many online soap recipe calculators assume the NaOH purity is 100%, but that is seldom true, even for high quality NaOH shipped fresh from the manufacturer.
The calculators' assumption of 100% purity builds in a "hidden" superfat in addition to the superfat (lye discount) percentage you type into the calculator when you create a recipe.
You can adjust your recipes to compensate for NaOH purity being less than 100% using a math calculation. This calculation is described in the "Step-by-step instructions..." file in the previous section. Use this method if you are calculating a soap recipe by hand.
If you are using an online soap recipe calculator that does not allow you to set the NaOH purity directly, here is a simple way to make this adjustment using the lye discount (superfat) setting:
Hidden superfat = 100% - Actual NaOH purity
Lye discount to type into the recipe calc = Actual superfat you want - Hidden superfat
Here are some examples --
The purity of my NaOH is 97%.
Hidden superfat = 100% - 97% = 3%
I want the actual superfat to be 5%.
Lye discount to type into the recipe calc = 5% - 3% = 2%.My NaOH is 95% pure.
Hidden superfat = 100% - 95% = 5%
I want the actual superfat to be 5%.
Lye discount to type into the recipe calc = 5% - 5% = 0%My NaOH is 75% pure.
Hidden superfat = 100% - 75% = 25%
I want the actual superfat to be 8%.
Lye discount to type into the recipe calc = 8% - 25% = -17%.
Yes, the number is negative -- you'd enter a minus 17 for the lye discount.
To adjust a recipe that uses KOH rather than NaOH: If your soap recipe calculator does not allow you to set the actual KOH purity, use this method to adjust for the actual KOH purity.
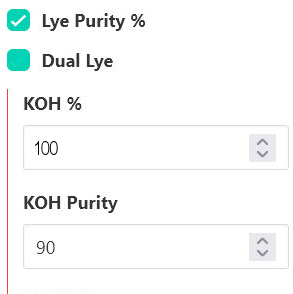
Some soap recipe calculators, such as LyeCalc, allow users to change the KOH purity, so there is no need for this adjustment. Just enter the KOH purity in the correct box:
NaOH and KOH are powerful desiccants, meaning they absorb water easily and quickly from the air. Here is a video using NaOH that makes this point:
See how fast NaOH absorbs water from the open air....
In the video, I use the word "deliquescence." Deliquescence is the name for the unusual ability of some chemicals, such as NaOH, to absorb so much water that the chemical turns from a solid into a liquid.
So what can we do to keep NaOH and KOH as pure as possible? A "dry bucket" as shown in this video is a homemade storage method that works well to protect your NaOH or KOH.
To summarize the video, smaller air-tight containers of NaOH and KOH are put inside a sturdy 5- or 7-gallon plastic bucket. A commercial desiccant (water absorbent chemical) is added to the large outer bucket to remove moisture from the air around the smaller alkali containers. The bucket is kept tightly covered with its original snap-on lid or a screw-on "gamma" lid.
A dry bucket has other important benefits. It will safely contain spills if an NaOH or KOH storage container leaks. It will also discourage children and pets from getting into these chemicals.
Caution: You will want to add a proven child-resistant closure for the best safety with children.
IMPORTANT: Do NOT put desiccant in direct contact with NaOH or KOH! The desiccant must go in the larger dry bucket OUTSIDE the alkali containers. It will NOT function properly if put directly in with the alkali.
Why won't the desiccant work this way? NaOH or KOH is a powerful desiccant in its own right -- MUCH more powerful than the safer chemicals commercially sold as desiccants, such as silica gel, calcium sulfate, calcium chloride, etc.
If put together in the same space, the NaOH will absorb any water out of the commercial desiccant as well as absorb any water vapor in the space. The "official" desiccant will never be functional as long as it is in the same space as NaOH or KOH.
So what's the point of having desiccant in the outer bucket? The smaller containers are the first and best defense to keep the alkali inside from absorbing moisture. No container is absolutely perfect, however. The desiccant dehumidifies the air in the larger bucket to add an extra layer of protection.
More discussion about the dry bucket storage method -- https://www.soapmakingforum.com/threads/my-lye-storage-setup.59316/
References
(1) Dunn, Kevin. Scientific Soapmaking. Clavicula Press. 2010. See especially Chapter 16: Caustic soda.
(2) Dunn, Kevin. Video lecture at http://fyi101.com/the-balancing-act-part-ii-presented-by-dr-kevin-dunn/
Copyright © 2002-2025 - All rights reserved by Classic Bells Ltd.
Template by OS-templates.com